MC1568
Cat. No.:YN290408
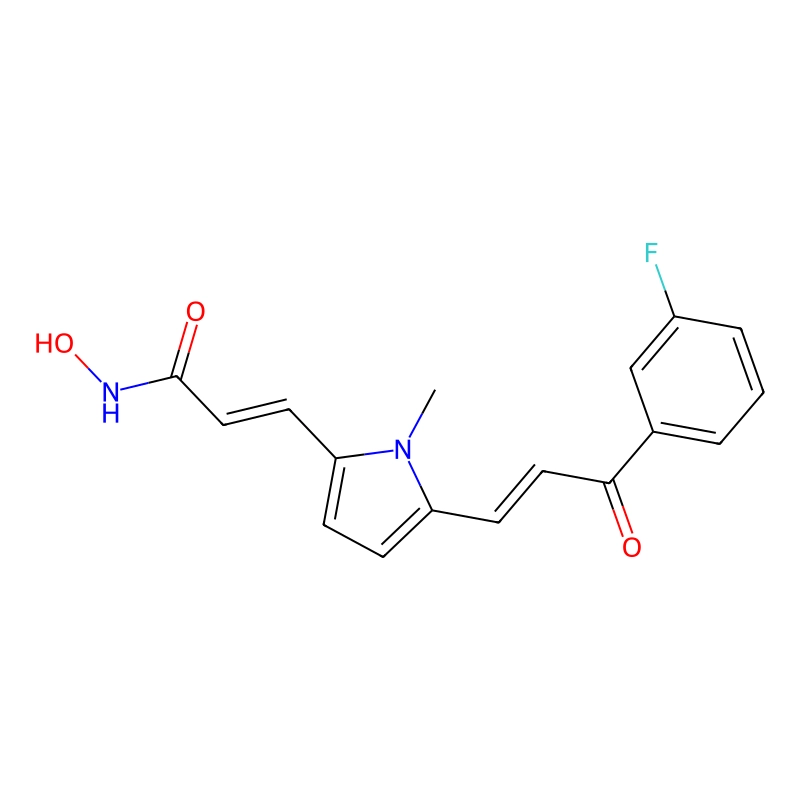
CAS No. :852475-26-4
| 产品名称: | MC1568 |
| CAS No.: | 852475-26-4 |
| Chemical Name: | (2E)-3-[5-[(1E)-3-(3-fluorophenyl)-3-oxo-1-propen-1-yl]-1-methyl-1H-pyrrol-2-yl]-N-hydroxy-2-propenamide |
| Synonyms: | MC1568;(E)-3-[4-[(E)-3-(3-fluorophenyl)-3-oxoprop-1-enyl]-1-methylpyrrol-2-yl]-N-hydroxyprop-2-enamide;MC-1568;MC1568 (MC-1568, MC 1568);(E)-3-(4-((E)-3-(3-fluorophenyl)-3-oxoprop-1-enyl)-1-methyl-1H-pyrrol-2-yl)-N-hydroxyacrylamide;MC-1568,MC1568;3-[5-(3 |
| 分子量: | 314.31 |
| 分子式: | C₁₇H₁₅FN₂O₃ |
| SMILES: | O=C(NO)/C=C/C1=CC=C(/C=C/C(C2=CC=CC(F)=C2)=O)N1C |
| 存储: | Please store the product under the recommended conditions in theCertificate of Analysis. |
| 运输: | Room temperature in continental US; may vary elsewhere. |
| 产品描述: | MC1568是组蛋白脱乙酰酶(HDAC II)的抑制剂,可用于癌症研究。 |
| IC50和靶点: | |
| In Vitro: | |
| In Vivo: | |
| Clinical Trial: | |
| Solvent & Solubility: |
de Ruijter, A.J., van Gennip, A.H., Caron, H.N., et al.Histone deacetylases (HDACs): Characterization of the classical HDAC familyBiochem J.370(Pt 3),737-749(2003)
Yang, X.J., and Grégoire, S.Class II histone deacetylases: From sequence to function, regulation, and clinical implicationMol. Cell. Biol.25(8),2873-2884(2005)
Mai, A., Massa, S., Pezzi, R., et al.Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamidesJ. Med. Chem.48(9),3344-3353(2005)
Mai, A., Jelicic, K., Rotili, D., et al.Identification of two new synthetic histone deacetylase inhibitors that modulate globin gene expression in erythroid cells from healthy donors and patients with thalassemiaMol. Pharmacol.72(5),1111-1123(2007)
Nebbioso, A., Manzo, F., Miceli, M., et al.Selective class II HDAC inhibitors impair myogenesis by modulating the stability and activity of HDAC-MEF2 complexesEMBO reports10(7),776-782(2009)
Duong, V., Bret, C., Altucci, L., et al.Specific activity of class II histone deacetylases in human breast cancer cellsMol. Cancer Res.6(12),1908-1919(2008)
Nebbioso, A., Dell'Aversana, C., Bugge, A., et al.HDACs class II-selective inhibition alters nuclear receptor-dependent differentiationJ. Mol. Endocrinol.45(4),219-228(2010)
Wang, G., He, J., Zhao, J., et al.Class I and class II histone deacetylases are potential therapeutic targets for treating pancreatic cancerPLoS One7(12),1-10(2012)
Mannaerts, I., Eysackers, N., Onyema, O.O., et al.Class II HDAC inhibition hampers hepatic stellate cell activation by induction of microRNA-29PLoS One8(1),1-9(2013)
Spallotta, F., Tardivo, S., Nanni, S., et al.Detrimental effect of class-selective histone deacetylase inhibitors during tissue regeneration following hindlimb ischemiaJ. Biol. Chem.288(32),22915-22929(2013)