Sorafenib
Cat. No.:YN350016
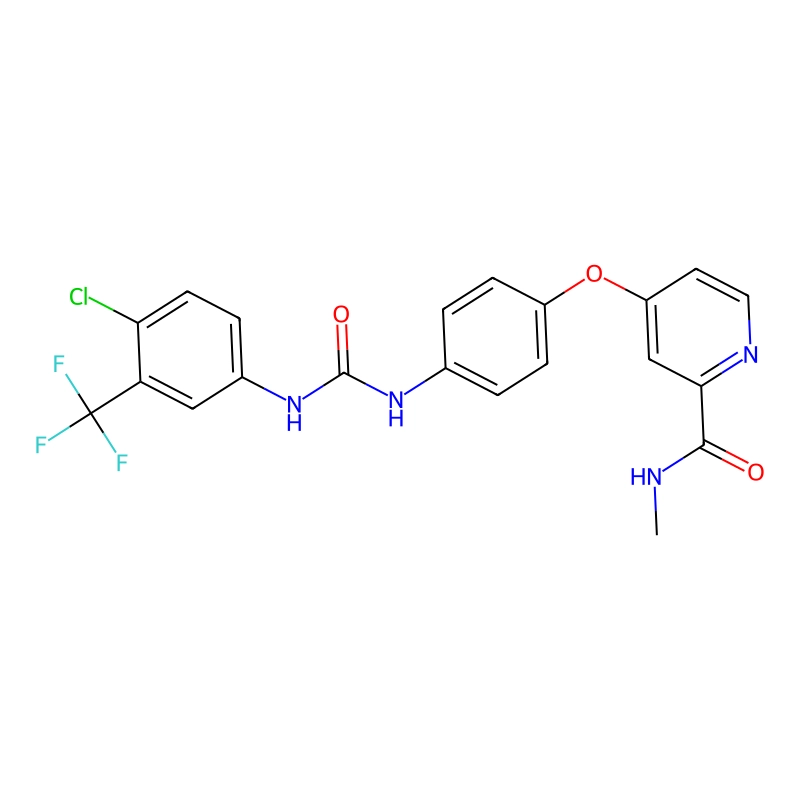
CAS No. :284461-73-0
| 产品名称: | Sorafenib |
| CAS No.: | 284461-73-0 |
| Chemical Name: | 4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-N-methyl-2-pyridinecarboxamide |
| Synonyms: | 索拉非尼; Bay 43-9006 |
| 分子量: | 464.83 |
| 分子式: | C₂₁H₁₆ClF₃N₄O₃ |
| SMILES: | O=C(NC(C=C1)=CC=C1OC2=CC(C(NC)=O)=NC=C2)NC3=CC=C(Cl)C(C(F)(F)F)=C3 |
| 存储: | Please store the product under the recommended conditions in theCertificate of Analysis. |
| 运输: | Room temperature in continental US; may vary elsewhere. |
| 产品描述: | Sorafenib (Bay 43-9006) 是一种有效的口服活性Raf抑制剂,对Raf-1和B-Raf的IC50分别为 6 nM 和 20 nM。Sorafenib 是一种多激酶抑制剂,对VEGFR2,VEGFR3,PDGFRβ,FLT3和c-Kit的IC50分别为 90 nM,15 nM,20 nM,57 nM 和 58 nM。Sorafenib 诱导细胞自噬 (autophagy) 和凋亡 (apoptosis),并具有抗肿瘤活性。Sorafenib 也是一种ferroptosis激动剂。 |
| IC50和靶点: | [{name:"VEGFR3:20 nM (IC50)"},{name: "Braf:22 nM (IC50)"},{name: "Raf-1:6 nM (IC50)"},{name: "VEGFR2:90 nM (IC50)"},{name: "PDGFRβ:57 nM (IC50)"},{name: "BrafV599E:38 nM (IC50)"},{name: "c-Kit:68 nM (IC50)"},{name: "Flt3:58 nM (IC50)"}] |
| In Vitro: | |
| In Vivo: | |
| Clinical Trial: | |
| Solvent & Solubility: |
Lyons, J.F., Wilhelm, S., Hibner, B., et al.Discovery of a novel Raf kinase inhibitorEndocr. Relat. Cancer8(3),219-225(2001)
Wilhelm, S.M., Carter, C., and Tang, L.BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesisCancer Res.64(19),7099-7109(2004)
Liu, L., Cao, Y., Chen, C., et al.Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5Cancer Res.66(24),11851-11858(2006)
Murphy, D.A., Makonnen, S., Lassoued, W., et al.Inhibition of tumor endothelial ERK activation, angiogenesis, and tumor growth by sorafenib (BAY43-9006)Am. J. Pathol.169(5),1875-1885(2006)
Dixon, S.J., Patel, D.N., Welsch, M., et al.Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosisElife3,e02523(2014)
Zheng, J., Sato, M., Mishima, E., et al.Sorafenib fails to trigger ferroptosis across a wide range of cancer cell linesCell Death Dis.12(7),698(2021)
Himmelsbach, K., Sauter, D., Baumert, T.F., et al.New aspects of an anti-tumour drug: Sorafenib efficiently inhibits HCV replicationGut58(12),1644-1653(2009)